Submitting Parasitology Specimens to the Lab
|
The DSHS Laboratory does not accept parasitology specimens from the public. Only healthcare providers can order parasitology tests and submit specimens for testing. Specimens submitted by the public will be rejected and destroyed. If you believe you have a parasitic infection, please talk with your healthcare provider. Laboratory staff cannot provide medical advice. Your provider will contact the DSHS Parasitology Laboratory for specimen submission requirements, if needed. |
- Parasitology Home | Parasite Testing at DSHS Laboratory | Refugee Health | Submitting Parasitology Specimens | Parasitology FAQs | Parasitology Resources -
Quick Links: Specimen Submission | Fecal/Stool | Worm Identification | Peripheral Blood Smears | EDTA Whole Blood | Urine | Miscellaneous Body Fluids | CDC Referral Specimens | Surveillance Specimen Specimens | Specimen Labeling Requirements | Completing Submission Forms: Best Practices | Shipping Parasitology Specimens to the DSHS Laboratory |
Specimen Submission
The steps to submitting parasitology specimens are similar to those of many other specimens sent to the Laboratory, as outlined in the graphic below.
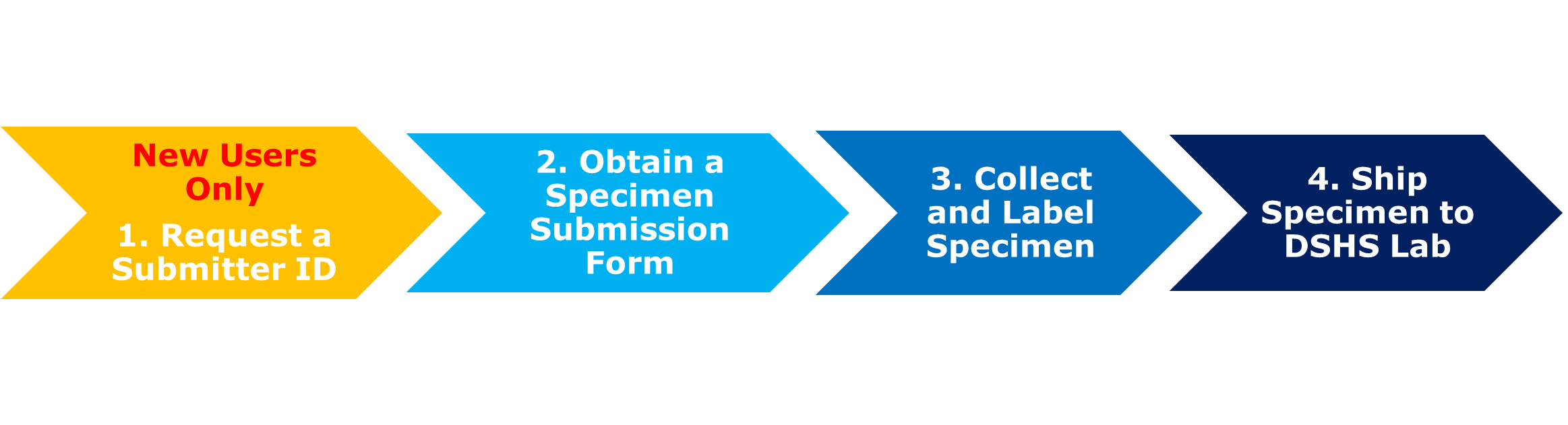
NOTE: This specimen submission guidance is intended for healthcare providers, clinical, regional, and reference labs only. The Laboratory does not accept parasitology submissions from the general public.
Clinical Specimen Submissions
Please refer to DSHS’ LTSM test menu for specific guidance on specimen types, required volumes, and shipping temperatures for your requested test. Refer to DSHS’ online Specimen Collection and Submission Guidance and Specimen Shipping and Mailing Guidance content for additional guidance on the required steps to submitting clinical specimens to the Laboratory.
Parasitology Specimens for Surveillance Programs
The Parasitology Laboratory collaborates with CDC on surveillance initiatives to sequence and genotype parasites responsible for foodborne illnesses and outbreaks. The gastrointestinal illnesses cyclosporiasis and cryptosporidiosis, caused by the parasites Cyclospora cayetanensis and Cryptosporidium spp. respectively, tend to be more prevalent during the summer months.
Genetic sequencing of Cyclospora and Cryptosporidium is essential to improving the public health response to future outbreaks and safeguard Texans against these foodborne parasites.
Free Cryptosporidium and Cyclospora Surveillance Testing
Submitting laboratories will not be charged for testing of specimens submitted to the DSHS Laboratory for Cryptosporidium and Cyclospora surveillance. A laboratory report will be issued to the submitter.
- For more information on tracking Cryptosporidium cases and outbreaks and the CryptoNet program, visit CDC’s CryptoNet webpage.
- For more information on tracking Cyclospora cayetanensis by genetic analysis, visit CDC’s advanced molecular detection of Cyclospora webpage.
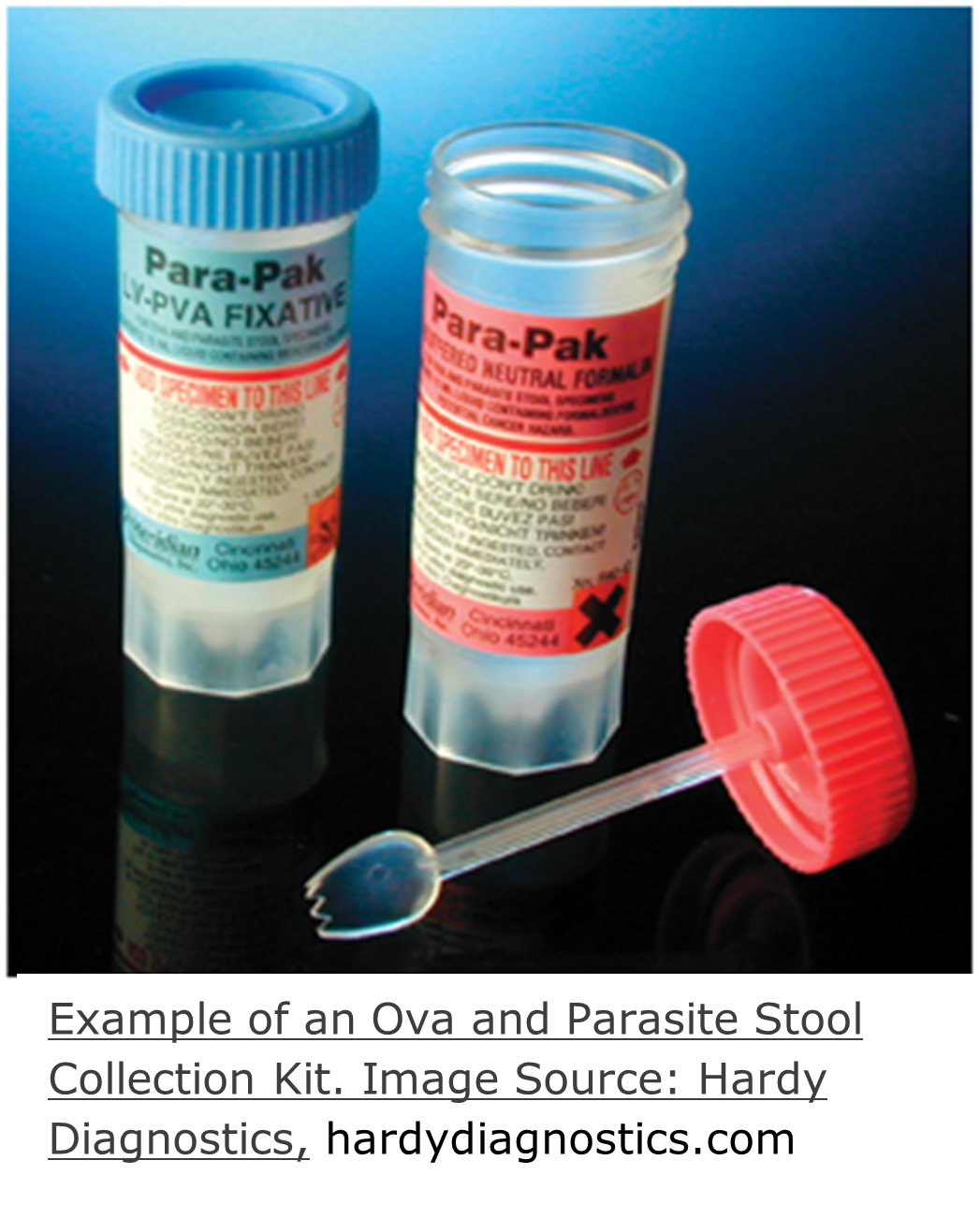 Fecal/Stool
Fecal/Stool
The Parasitology Lab accepts fecal specimens provided in Ova and Parasite (O&P) kits, fixed in 10% formalin and LV-PVA.
- Stool specimens must be adequately mixed in fixative.
- A minimum volume of 5ml is required.
- Avoid overfilling specimen containers.
The submission of raw/unpreserved fecal specimens or rectal swabs may result in a “compromised specimen” note being included in the final report. The absence of preservatives in specimens can reduce the accuracy of parasite detection, as they may deteriorate or undergo morphological changes in the sample.
Please ensure collection kits are not expired. Specimens collected and submitted in expired vials will be rejected upon arrival.
Worm Identification
Submissions of suspected helminths (worms) retrieved from the intestinal tract, body tissues, or stool are accepted for testing. Specimens must be fixed in 70% ethanol (preferred) or 10% formalin.
Peripheral Blood Smears
Submission of thick and thin blood smears are accepted for Giemsa staining of bloodborne protozoa species. The smears may be either pre-stained or unstained by submitter.
- Prepare and submit at least two thick and thin smears per patient.
- Ensure smears are fully dry before enclosing in slide carrier.
- Submit smears with an EDTA whole blood specimen (lavender/purple top tube).
- Ensure a minimum volume of approximately 1 mL for testing purposes.
Additional information on blood collection and blood smear preparation is available at CDC: CDC - DPDx - Diagnostic Procedures - Blood Specimens
EDTA Whole Blood
Whole blood specimens are accepted for Giemsa staining and morphological examination. EDTA whole blood is preferred.
- Collect specimen in EDTA (lavender/purple top) tubes.
- Minimum volume of 1 mL required.
- Refrigerate specimens and ship cold as soon as possible after collection.
Urine
Urine specimens are accepted for staining and morphological examination.
- Collect specimen in sterile container.
- Minimum volume of 10 mL required.
- Refrigerate specimens and ship cold.
Miscellaneous Body Fluids
A variety of body fluid specimens are accepted for staining and microscopic morphological examination. Body fluids are specimens other than stool, plasma, serum, or urine.
- Storage and preservation are dependent upon fluid type/origin.
- Call Medical Parasitology at 512-776–7560 for instructions on the preservation and handling of specimen(s) for submission.
- Bodily fluids or tissue containing suspected helminths/parasites may be forwarded to the CDC at the discretion of the Parasitology Team for further analysis.
CDC Referral Specimens
All CDC referral specimens should be sent to the DSHS Austin Laboratory. Providers should refrain from submitting specimens directly to the CDC unless they have obtained prior approval from the CDC to do so. For time-sensitive cases, please contact the Parasitology Team at 512-776-7560 for guidance.
Surveillance Program Specimens
DSHS collaborates with CDC in parasite surveillance programs such as CryptoNet and Cyclospora genotyping for the collection and molecular characterization of Cryptosporidium species and Cyclospora. Clinical laboratories are highly encouraged to submit Cryptosporidium and Cyclospora positive stool specimens to DSHS for molecular analysis and/or forwarding to CDC for identification and sequencing of the organisms. This information can be valuable in linking cases during outbreak investigations.
Cyclospora Genotyping Specimens
- Required Media Stool specimens fixed with Zn-PVA, Cu-PVA or Ecofix (or other parasitology fixative without formalin) are acceptable. Raw stool and unfixed specimens (e.g., collected in Cary-Blair transport media) are also acceptable.
Please note: Specimens fixed in LV-PVA are not acceptable as the formalin it contains interferes with the testing process. - Required Volume A minimum of 300 µl
- Required Shipping Temperature Fixed samples may be stored and shipped at room temperature. Unfixed (i.e., Cary-Blair) and raw stool specimens should be kept refrigerated at 2-8 °C and be shipped overnight in insulated containers with cold packs. Do not use dry ice.
Cryptosporidium spp. Genotyping Specimens
- Required Media Stool specimens fixed with Zn-PVA, Cu-PVA or Ecofix (or other parasitology fixative without formalin) are acceptable. Raw stool and unfixed specimens (e.g., collected in Cary-Blair transport media) are also acceptable.
Please note: Specimens fixed in LV-PVA are not acceptable as the formalin it contains interferes with the testing process. - Required Volume A minimum of 300 µl
- Required Shipping Temperature Fixed samples may be stored and shipped at room temperature. Unfixed (i.e., Cary-Blair) and raw stool specimens should be kept refrigerated at 2-8 °C and be shipped overnight in insulated containers with cold packs. Do not use dry ice.
Specimen Labeling Requirements
The DSHS Laboratory requires primary specimen containers be labeled with at least two identifiers at the time of collection. The following specimen labeling/submission requirements must be met for all specimens submitted to the DSHS Laboratory, otherwise the specimens will not be tested.
- Every specimen must have at least two unique patient identifiers affixed to it. Three identifiers are preferred.
- Every specimen must be submitted with a DSHS submission form.
- Patient-specific identifiers on the specimen and the submission form must match exactly.
- All required fields in the forms must be completed.
Refer to the DSHS’ LTSM specimen labeling guidance for additional specimen labeling requirements.
Completing Submission Forms: Best Practices
Depending on the test being requested, a G-2A or G-2B submission form is required for every parasitology specimen submission. Submitters must ensure they are using the correct submission form for the test being requested. Every submission form must have the required patient identifiers and a date of collection. The form must also identify the specimen source, the test being requested, and the payor source.
Providing a completed specimen submission form with each specimen is essential to successful testing.
- If filling out the form by hand, please use only BLOCK lettering.
- Avoid using cursive script, which can cause legibility issues and transcription errors.
- Refer to the DSHS LTSM specimen collection guidance pages found here for more submission form guidance.
Shipping Parasitology Specimens to the DSHS Laboratory
Specimens submitted to the Laboratory for parasitology testing are classified as infectious substances. To minimize the likelihood of exposing mail handlers and carriers, specimens must be shipped to the laboratory according to Category B Biological Substance, UN3373 requirements. As a submitter, you have legal responsibilities regarding the handling, labeling, and shipping of Biological Substances, Category B UN3373.
Additional information on shipping infectious substances to the DSHS Austin Laboratory may be found at the laboratory’s general specimen shipping and mailing guidance pages.
Before shipping specimens, make sure you have
- labeled the specimen with the required patient identifiers (two minimum, three are preferred),
- secured the lid of the specimen container to prevent leakage,
- completed the correct specimen submission form for the specimen and requested test,
- provided the date of collection on the submission form,
- selected the test being requested on the submission form,
- attached a copy of previous lab results, if available,
- packed the specimen correctly according to Category B, UN3373 shipping requirements,
- affixed all required shipping labels to the outer mailer, and
- packed cold packs, not dry ice with chilled specimens when applicable.
Questions about Parasitology Submissions?
For questions about parasitology specimen submissions to the DSHS Laboratory or CDC, contact the Parasitology Team at 512-776-7560, or email Medical.Parasitology@dshs.texas.gov.